Friedrich et al. 2023
High-resolution brain atlas for deep brain stimulation imaging
Mesoscale Brain Mapping Symposium 2023 | Poster Abstract
A High Resolution Brain Atlas for Deep Brain Stimulation Imaging
Helen Friedrich1,2, Simon Oxenford3, Eduardo J. L. Alho4, Brian L. Edlow5,6, Clemens Neudorfer1,3,7, Andreas Horn1,3,5,7
Contact: hfriedrich@bwh.harvard.edu; ahorn1@bwh.harvard.edu
1 Center for Brain Circuit Therapeutics Department of Neurology Brigham & Women’s Hospital, Harvard Medical School, Boston MA 02115, USA
2 Julius-Maximilian-University Wuerzburg, Germany
3 Movement Disorder and Neuromodulation Unit, Department of Neurology, Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt- Universität zu Berlin, Department of Neurology, 10117 Berlin, Germany
4 Clinic of Pain and Functional Neurosurgery, São Paulo, Brazil
5 Center for Neurotechnology and Neurorecovery, Department of Neurology, Massachusetts General Hospital, Boston, MA 02114, USA
6 Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, USA
7 Brain Modulation Lab, Department of Neurosurgery, Massachusetts General Hospital, Boston, MA, 02114, USA
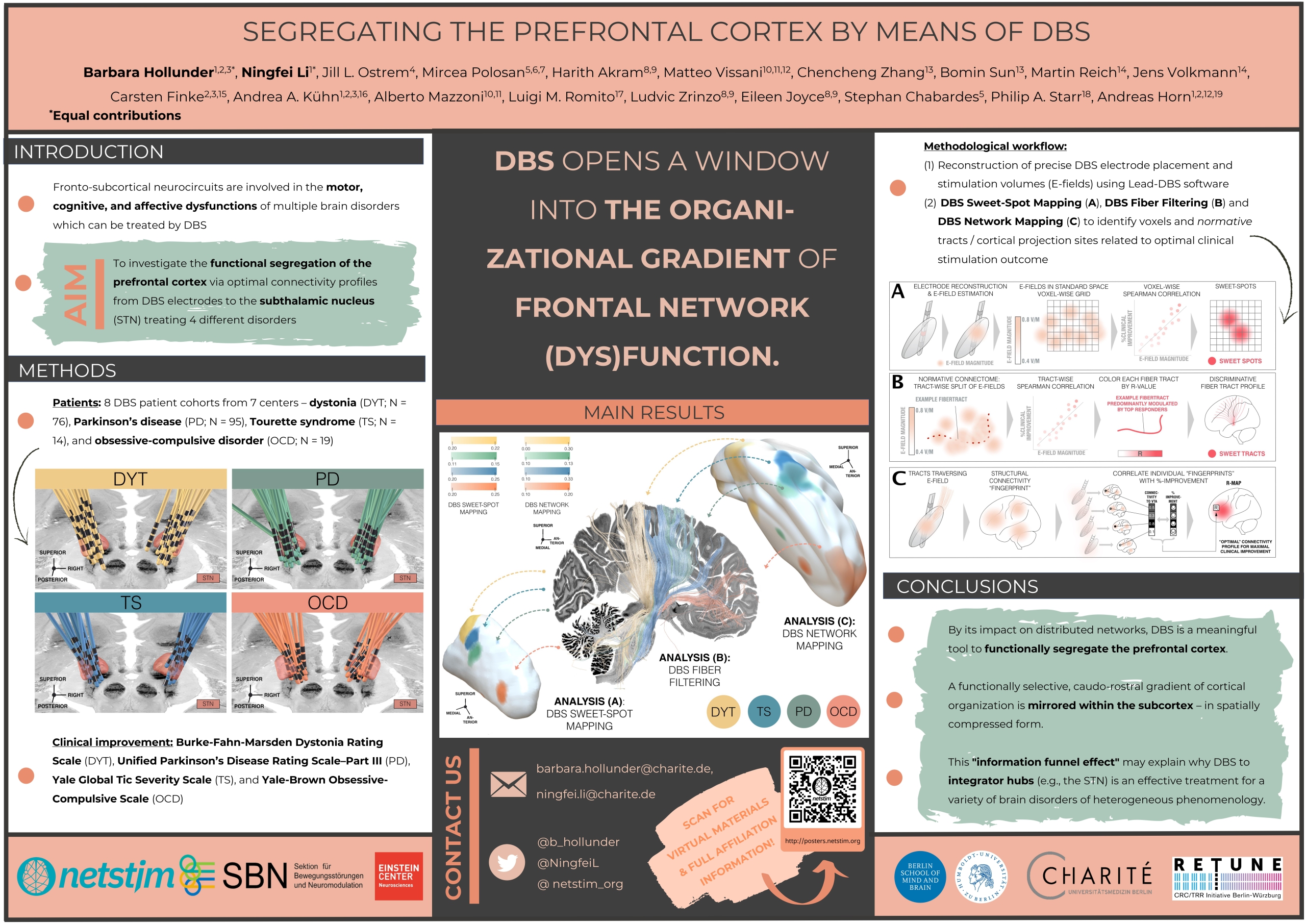
Background
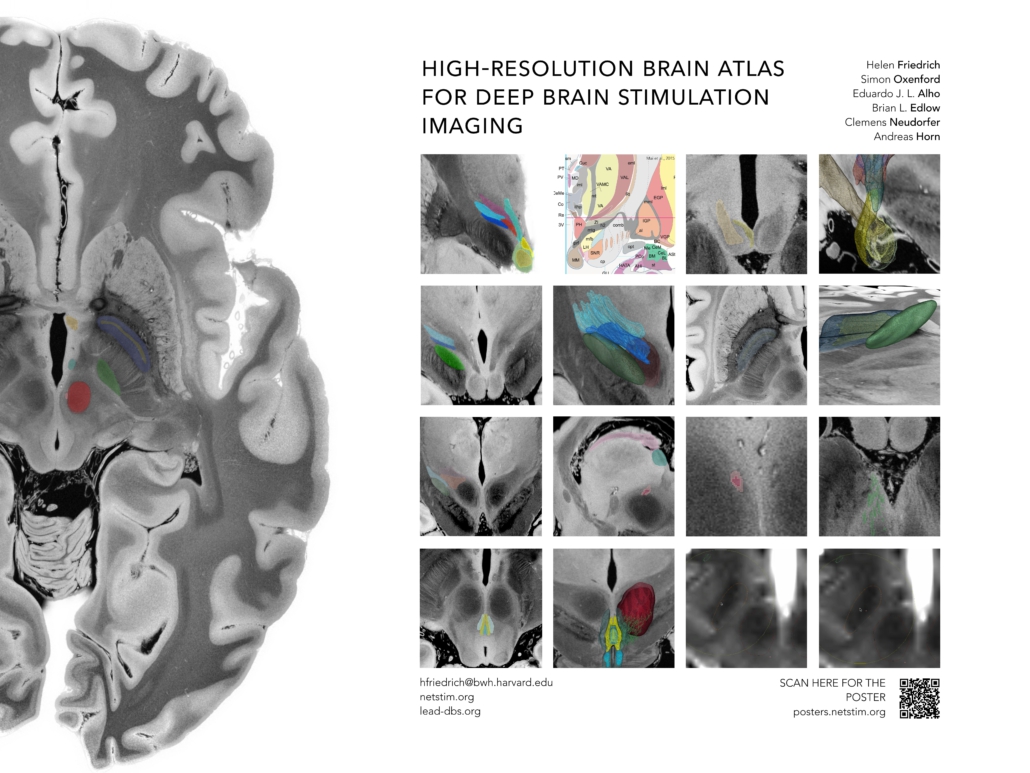
The success of deep brain stimulation therapy is defined by the exact placement of electrodes in relation to their targets. Overlaps between stimulation volumes and anatomical structures may account for clinical outcomes. To investigate this, precise definitions of anatomical structures are critical. These target structures frequently comprise anatomical structures or tracts that are often not discernable on conventional neuroimaging.
Methods
We aimed to create a high-resolution atlas including both grey and white matter based on a 100-micron ex-vivo MRI brain scan (Edlow et al., 2019). Histological data, postmortem fiber dissections, and expert opinions of clinicians and anatomists were used to inform and enrich anatomical validity. Segmentations of gray- (n=15) and white- (n=16) matter structures at the level of the subthalamus were carried out on the high resolution MRI scan and converted into MNI ICBM 2009b NLIN ASYM space. This spatial transform from MRI native to template space was carried out using a manually curated warpfield that accounts for proper definitions of target structures in standard space.
Results & Conclusions
The main result of this work should be seen in a high-resolution atlas of the human subthalamus comprising a total of 31 gray and white matter regions per hemisphere. Together with the ex-vivo MRI template and a precise manual registration to MNI space, this dataset is deformable to patient MRIs and can be readily applied in DBS imaging studies.
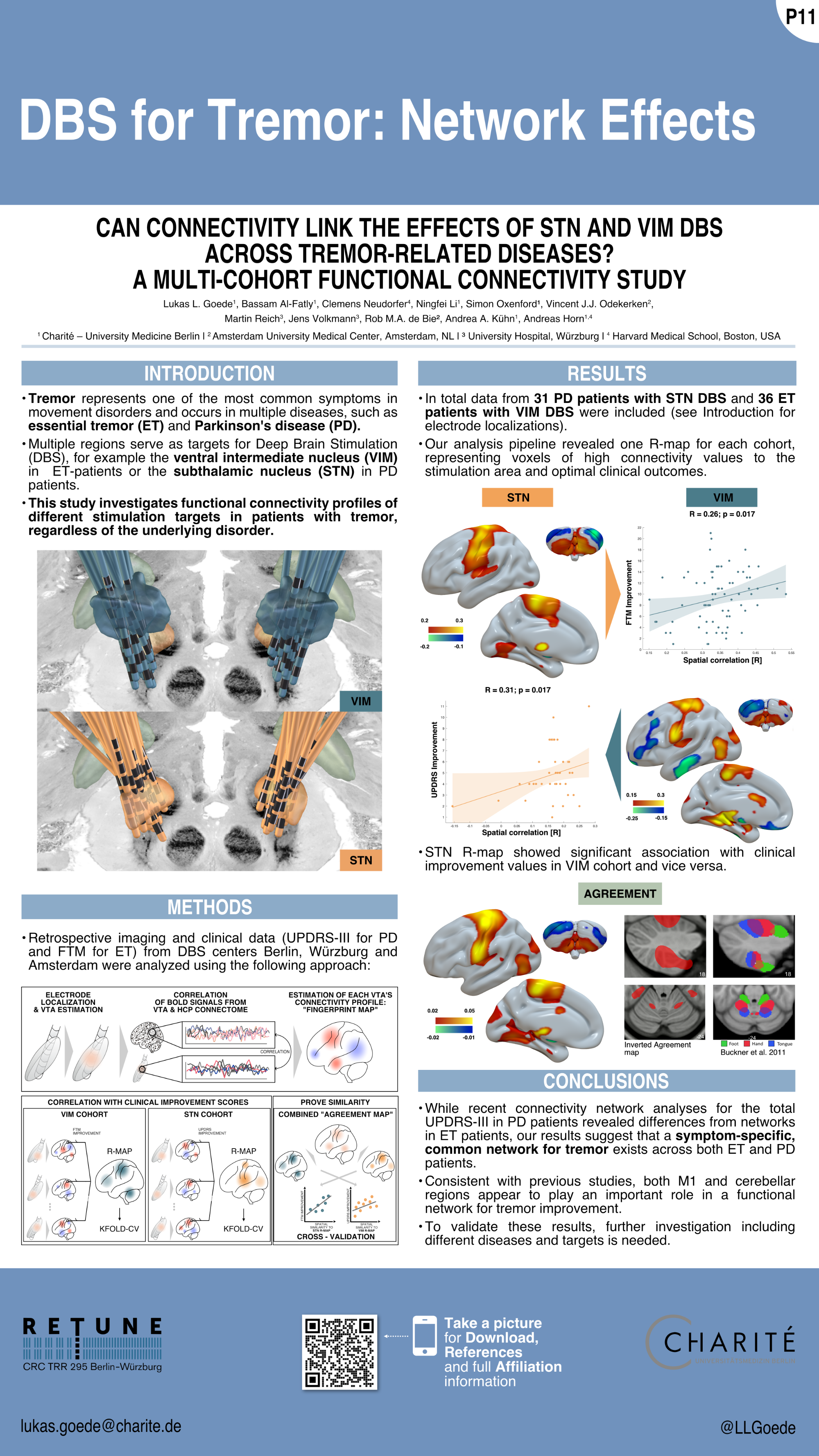
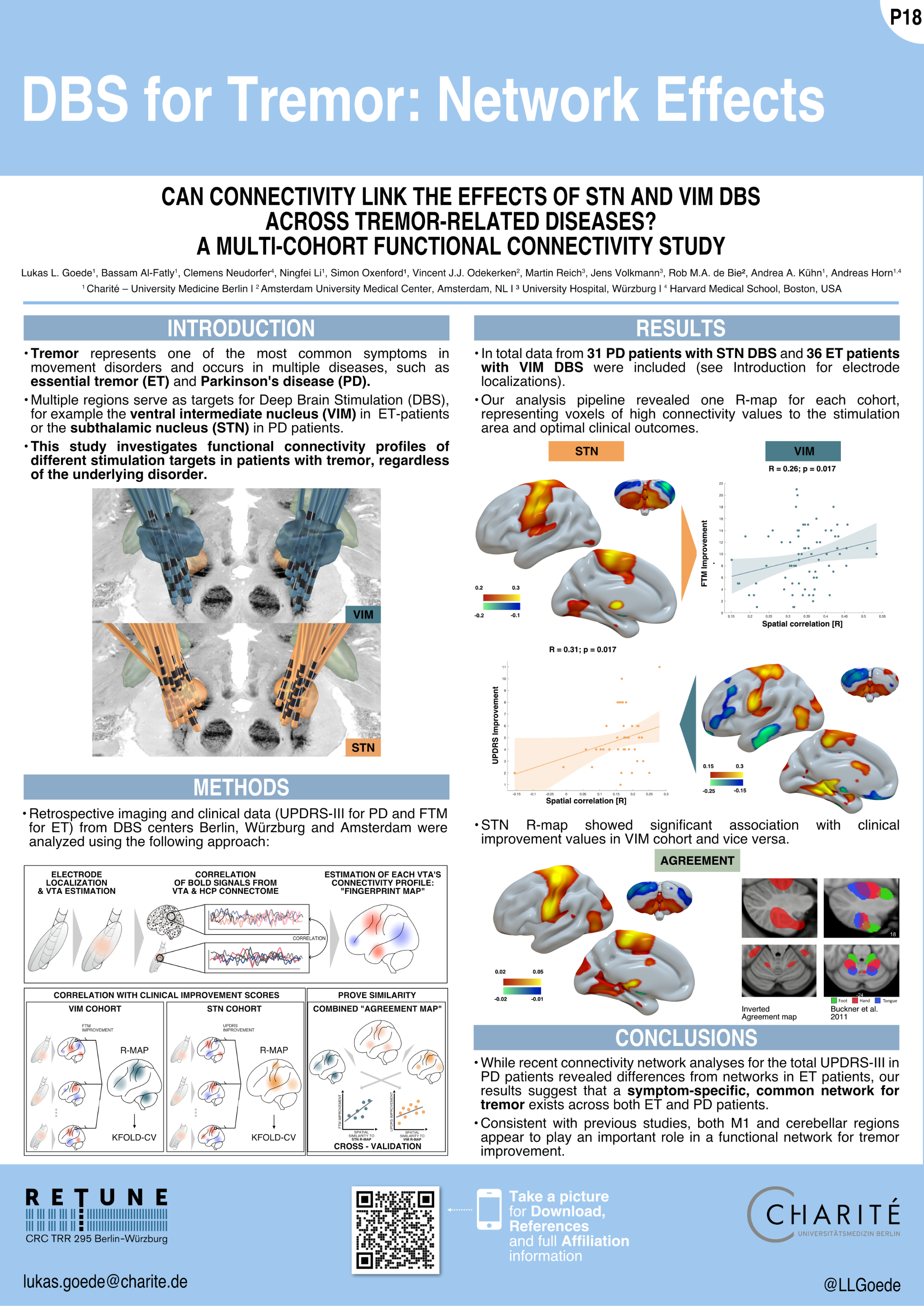
Goede et al. (MDS 2023)
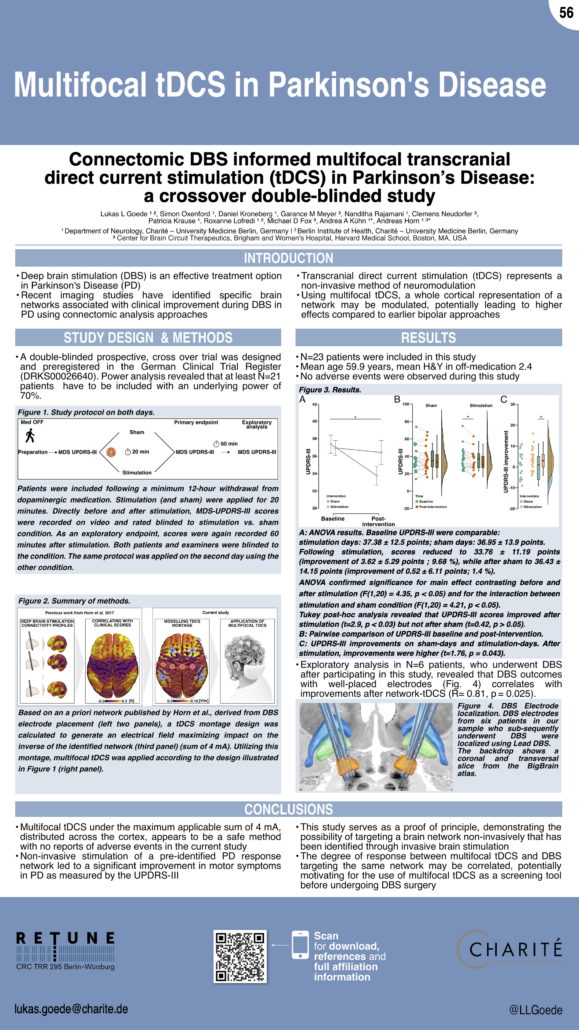
Connectomic DBS informed multifocal transcranial direct current stimulation (tDCS) in Parkinson’s Disease:
a crossover double-blinded study
Affiliations
Department of Neurology with Experimental Neurology, Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany
Berlin Institute of Health at Charité – Universitätsmedizin Berlin, BIH Biomedical Innovation Academy, BIH Charité Junior Clinician Scientist Program, Berlin, Germany
Center for Brain Circuit Therapeutics, Departments of Neurology, Psychiatry, and Radiology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA
MGH Neurosurgery & Center for Neurotechnology and Neurorecovery (CNTR) at MGH Neurology Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA
Bernstein Center for Computational Neuroscience, Humboldt-Universität, Berlin, Germany
NeuroCure, Exzellenzcluster, Charité-Universitätsmedizin Berlin, Berlin, Germany
DZNE, German Center for Neurodegenerative Diseases, Berlin, Germany
Berlin School of Mind and Brain, Humboldt-Universität zu Berlin, Berlin, Germany
Dr. Goede is participant in the BIH Charité Junior Clinician Scientist Program
funded by the Charité – Universitätsmedizin Berlin, and the Berlin Institute of Health at Charité (BIH).
Dr. Lofredi is participant in the BIH Charité Clinician Scientist Program
funded by the Charité – Universitätsmedizin Berlin, and the Berlin Institute of Health at Charité (BIH).
Funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Project-ID 424778381 – TRR 295 and Emmy Noether Stipend 410169619 to A.H.
References
[1] Fox MD, Buckner RL, Liu H, Chakravarty MM, Lozano AM, Pascual-Leone A. Resting-state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases. Proceedings of the National Academy of Sciences. 2014;111:E4367–E4375.
[2] Horn A, Reich M, Vorwerk J, et al. Connectivity Predicts deep brain stimulation outcome in Parkinson disease: DBS Outcome in PD. Ann Neurol. 2017;82:67–78.
[3] Sobesky L, Goede L, Odekerken VJJ, et al. Subthalamic and pallidal deep brain stimulation: are we modulating the same network? Brain. 2022;145:251–262.
[4] Fischer DB, Fried PJ, Ruffini G, et al. Multifocal tDCS targeting the resting state motor network increases cortical excitability beyond traditional tDCS targeting unilateral motor cortex. NeuroImage. 2017;157:34–44.
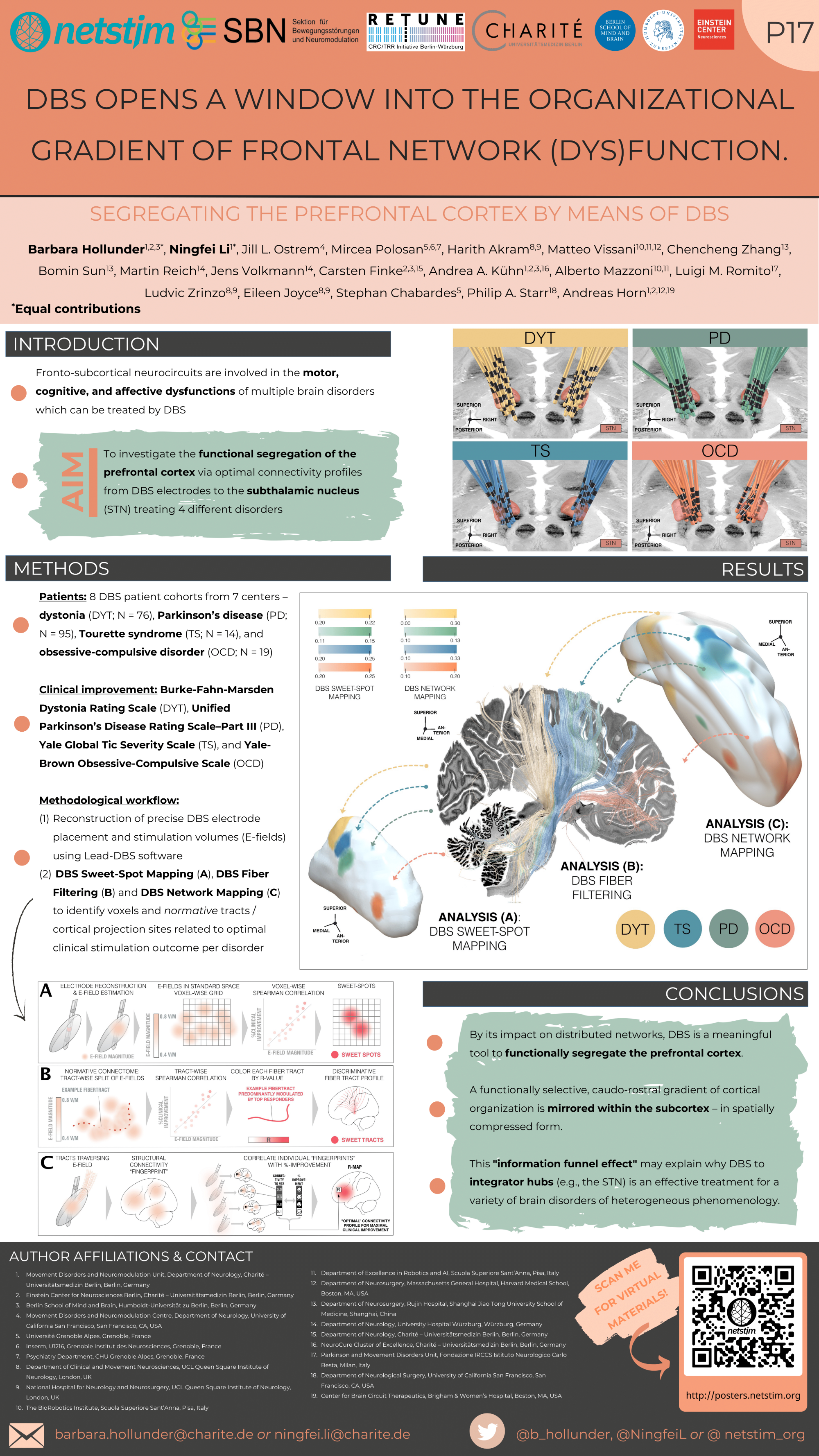
Hollunder et al. (OHBM 2023)
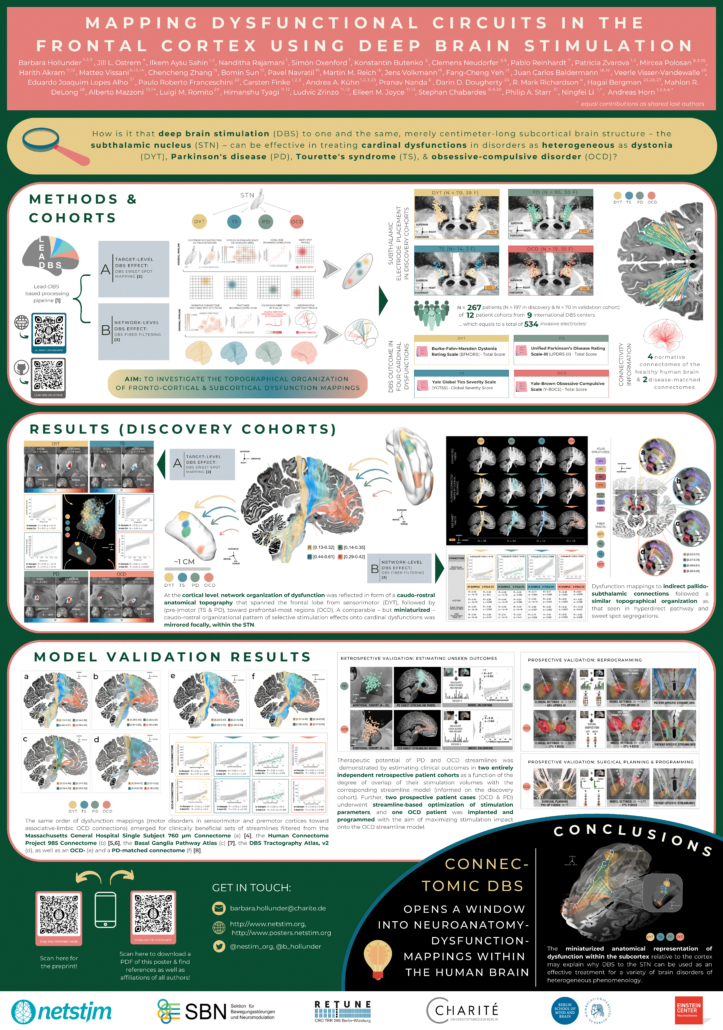
Mapping Dysfunctional Circuits in the Frontal Cortex Using Deep Brain Stimulation
Affiliations
- Department of Neurology, Charité – Universitätsmedizin Berlin, Berlin, Germany
- Einstein Center for Neurosciences Berlin, Charité – Universitätsmedizin Berlin, Berlin, Germany
- Berlin School of Mind and Brain, Humboldt-Universität zu Berlin, Berlin, Germany
- Movement Disorders and Neuromodulation Centre, Department of Neurology, University of California San Francisco, San Francisco, CA, USA
- Center for Brain Circuit Therapeutics, Department of Neurology, Brigham & Women’s Hospital, Harvard Medical School, Boston, MA, USA
- Department of Neurosurgery, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA
- Department of Psychiatry and Psychotherapy, Charité – Universitätsmedizin Berlin, Berlin, Germany
- Grenoble Alpes, Grenoble, France
- Inserm, U1216, Grenoble Institut des Neurosciences, Grenoble, France
- Psychiatry Department, Centre Hospitalier Universitaire Grenoble Alpes, Grenoble, France
- Department of Clinical and Movement Neurosciences, University College London Queen Square Institute of Neurology, London, UK
- National Hospital for Neurology and Neurosurgery, University College London Queen Square Institute of Neurology, London, UK
- The BioRobotics Institute, Scuola Superiore Sant’Anna, Pisa, Italy
- Department of Excellence in Robotics and AI, Scuola Superiore Sant’Anna, Pisa, Italy
- Department of Neurosurgery, Rujin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- Department of Neurology, University Hospital Würzburg, Würzburg, Germany
- Department of Bioengineering, University of Pittsburgh, Pittsburgh, PA, USA
- Department of Psychiatry and Psychotherapy, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany
- Department of Neurology, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany
- Department of Stereotactic and Functional Neurosurgery, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany
- Clinic of Pain and Functional Neurosurgery, São Paulo, Brazil
- Department of Neurology and Neurosurgery, University of Caxias do Sul, Rio Grande do Sul, Brazil
- NeuroCure Cluster of Excellence, Charité – Universitätsmedizin Berlin, Berlin, Germany
- Department of Psychiatry, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA
- The Edmond and Lily Safra Center for Brain Sciences, The Hebrew University, Jerusalem, Israel
- Department of Medical Neurobiology, Institute of Medical Research Israel-Canada, The Hebrew University, Hassadah Medical School, Jerusalem, Israel
- Department of Neurosurgery, Hadassah Medical Center, Jerusalem, Israel
- Department of Neurology, Emory University School of Medicine, Atlanta, GA, USA
- Parkinson and Movement Disorders Unit, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milan, Italy
- Department of Neurosurgery, Centre Hospitalier Universitaire Grenoble Alpes, Grenoble, France
- Department of Neurological Surgery, University of California San Francisco, San Francisco, CA, USA
References
[1] Neudorfer, C. et al. Lead-DBS v3.0: Mapping deep brain stimulation effects to local anatomy and global networks. Neuroimage 268, 119862 (2023).
[2] Horn, A. et al. Optimal deep brain stimulation sites and networks for cervical vs. generalized dystonia. Proc. Natl. Acad. Sci. 119, e2114985119 (2022).
[3] Irmen, F. et al. Left prefrontal connectivity links subthalamic stimulation with depressive symptoms. Ann. Neurol. 87, 962–975 (2020).
[4] Wang, F. et al. In vivo human whole-brain Connectom diffusion MRI dataset at 760 µm isotropic resolution. Sci. Data8, 122 (2021).
[5] Van Essen, D. C. et al. The WU-Minn Human Connectome Project: An overview. Neuroimage 80, 62–79 (2013).
[6] Li, N. et al. A unified connectomic target for deep brain stimulation in obsessive-compulsive disorder. Nat. Commun. 11, 3364 (2020).
[7] Petersen, M. V et al. Holographic reconstruction of axonal pathways in the human brain. Neuron 104, 1056-1064.e3 (2019).
[8] Ewert, S. et al. Toward defining deep brain stimulation targets in MNI space: A subcortical atlas based on multimodal MRI, histology and structural connectivity. Neuroimage 170, 271–282 (2018).
Meyer et al. (OHBM 2023)
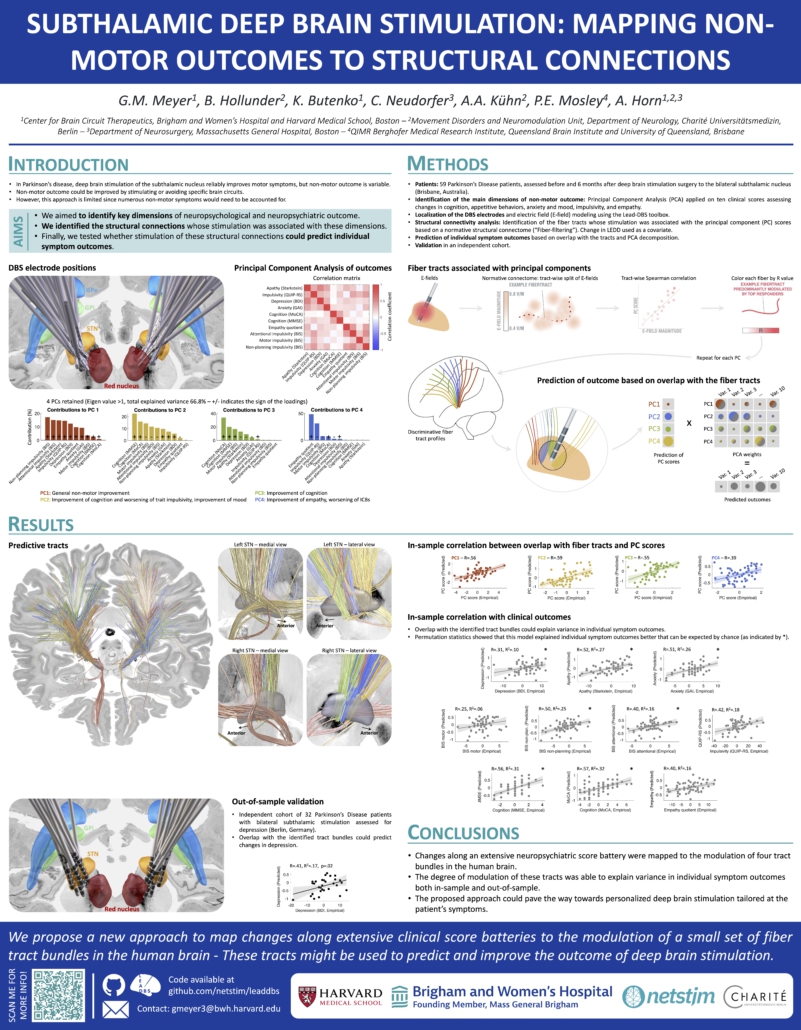
Subthalamic Deep Brain Stimulation: Mapping Non-Motor Outcomes to Structural Connections
References
[1] Irmen, F. et al. 2020. Left Prefrontal Connectivity Links Subthalamic Stimulation with Depressive Symptoms. Annals of Neurology 87, 962–975.
[2] Mosley, P.E. et al. 2020. The structural connectivity of subthalamic deep brain stimulation correlates with impulsivity in Parkinson’s. Brain 143, 2235–2254.
[3] Neudorfer, C. et al. 2023. Lead-DBS v3.0: Mapping deep brain stimulation effects to local anatomy and global networks. NeuroImage 268, 119862.
Kim et al. (DBS Society Congress 2023)
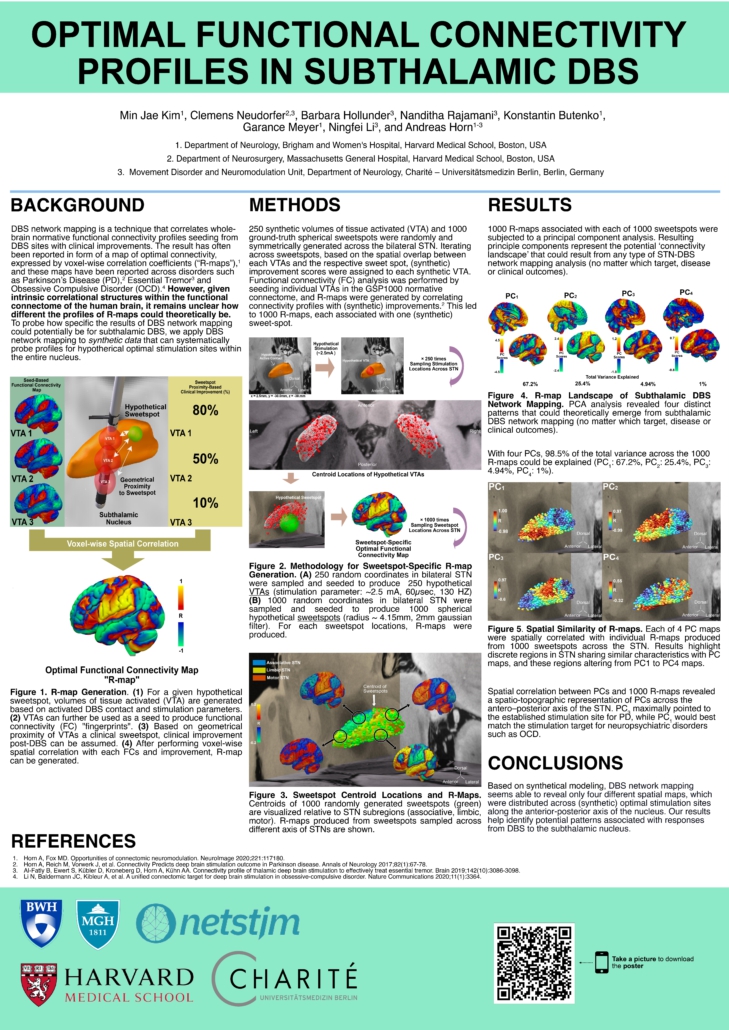
Optimal Functional Connectivity Profiles in Subthalamic DBS
References
[1] Horn A, Fox MD. Opportunities of connectomic neuromodulation. NeuroImage 2020;221:117180.
[2] Horn A, Reich M, Vorwerk J, et al. Connectivity Predicts deep brain stimulation outcome in Parkinson disease. Annals of Neurology 2017;82(1):67-78.
[3] Al-Fatly B, Ewert S, Kübler D, Kroneberg D, Horn A, Kühn AA. Connectivity profile of thalamic deep brain stimulation to effectively treat essential tremor. Brain 2019;142(10):3086-3098.
[4] Li N, Baldermann JC, Kibleur A, et al. A unified connectomic target for deep brain stimulation in obsessive-compulsive disorder. Nature Communications 2020;11(1):3364. (2018).
Sahin et al. (DBS Society Congress 2023)
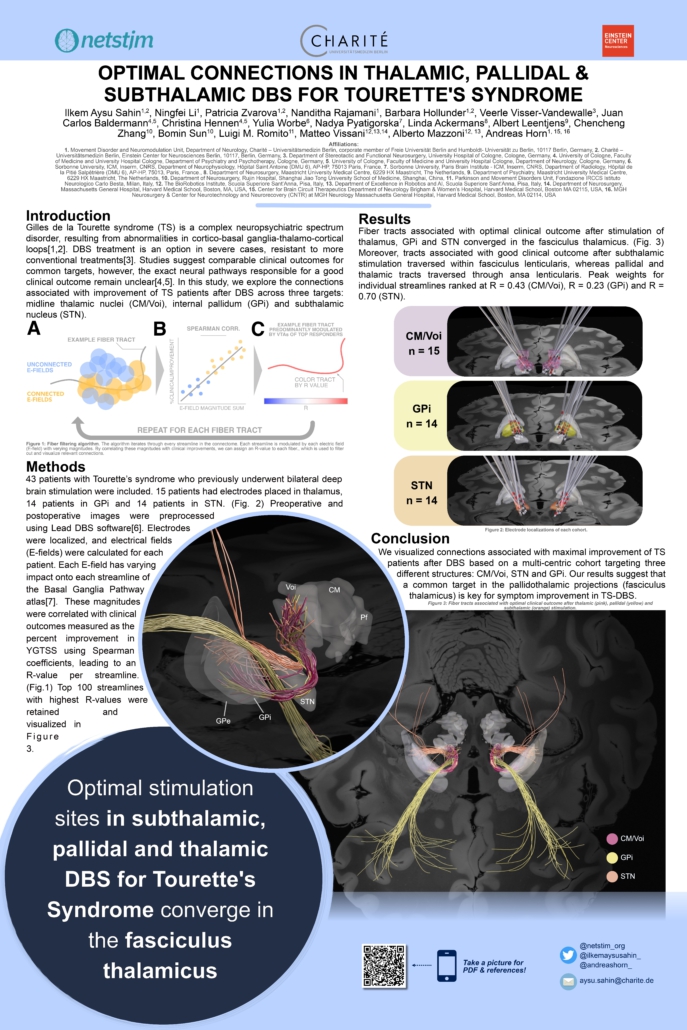
Optimal Connections in Thalamic, Pallidal and Subthalamic DBS for Tourette’s Syndrome
References
[1] Bronfeld, M. & Bar-Gad, I. Tic Disorders: What Happens in the Basal Ganglia? Neuroscientist 19, 101–108 (2013).
[2] Augustine, F. & Singer, H. S. Merging the Pathophysiology and Pharmacotherapy of Tics. Tremor Other Hyperkinet Mov (N Y) 8, 595 (2019).
[3] Billnitzer, A. & Jankovic, J. Current Management of Tics and Tourette Syndrome: Behavioral, Pharmacologic, and Surgical Treatments. Neurotherapeutics 17, 1681–1693 (2020).
[4] Martinez-Ramirez, D. et al. Efficacy and Safety of Deep Brain Stimulation in Tourette Syndrome: The International Tourette Syndrome Deep Brain Stimulation Public Database and Registry. JAMA Neurology 75, 353–359 (2018).
[5] Baldermann, J. C. et al. Deep Brain Stimulation for Tourette-Syndrome: A Systematic Review and Meta-Analysis. Brain Stimulation: Basic, Translational, and Clinical Research in Neuromodulation 9, 296–304 (2016).
[6] Neudorfer, C. et al. Lead-DBS v3.0: Mapping deep brain stimulation effects to local anatomy and global networks. NeuroImage 268, 119862 (2023).
[7] Petersen, M. V. et al. Holographic Reconstruction of Axonal Pathways in the Human Brain. Neuron 104, 1056-1064.e3 (2019).
Hollunder et al. (OHBM & DBS Society Congress 2023)
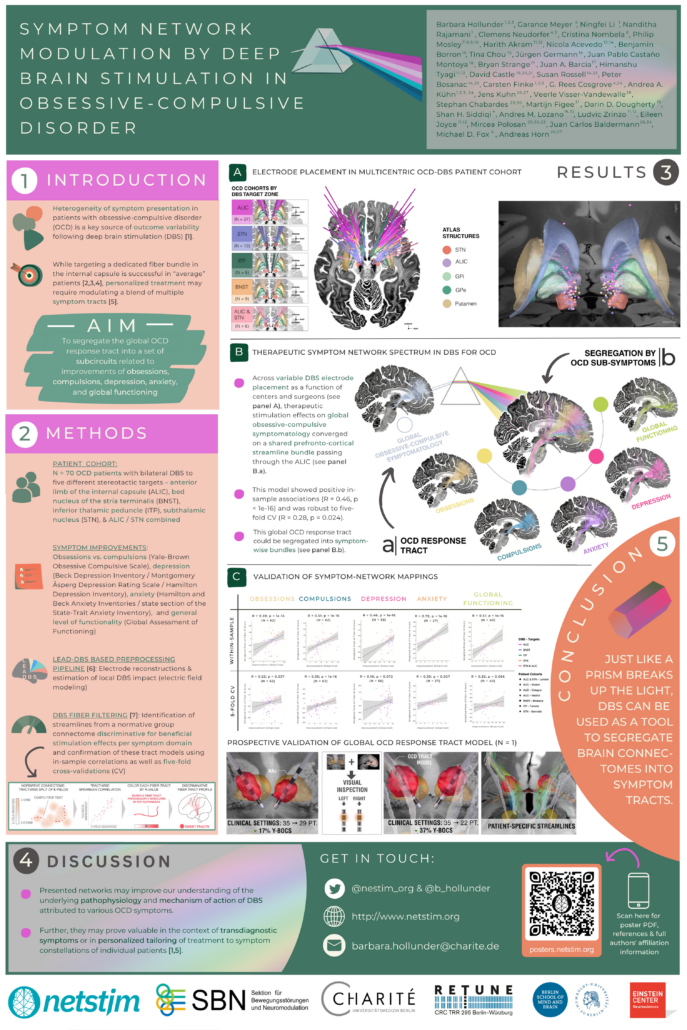
Symptom Network Modulation of Deep Brain Stimulation in Obsessive-Compulsive Disorder
Affiliations
- Department of Neurology, Charité – Universitätsmedizin Berlin, Berlin, Germany
- Einstein Center for Neurosciences Berlin, Charité – Universitätsmedizin Berlin, Berlin, Germany
- Berlin School of Mind and Brain, Humboldt-Universität zu Berlin, Berlin, Germany
- Center for Brain Circuit Therapeutics, Department of Neurology, Brigham & Women’s Hospital, Harvard Medical School, Boston, MA, USA
- Department of Neurosurgery, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA
- Biological and Health Psychology, School of Psychology, Universidad Autónoma de Madrid, Spain
- Clinical Brain Networks Group, QIMR Berghofer Medical Research Institute, Herston, Queensland, Australia
- Neurosciences Queensland, St Andrew’s War Memorial Hospital, Spring Hill, Queensland, Australia
- Queensland Brain Institute, University of Queensland, St Lucia, Queensland, Australia
- Australian eHealth Research Centre, CSIRO Health and Biosecurity, Herston, Queensland, Australia
- Department of Clinical and Movement Neurosciences, UCL Queen Square Institute of Neurology, London, UK
- National Hospital for Neurology and Neurosurgery, UCL Queen Square Institute of Neurology, London, UK
- Centre for Mental Health, Swinburne University of Technology, Melbourne, VIC, Australia
- Vincent’s Hospital Melbourne, Melbourne, Australia
- Department of Psychiatry, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA
- Division of Neurosurgery, Department of Surgery, University of Toronto, Toronto, ON, Canada
- Department of Neurosurgery, Hospital Clinico San Carlos, Instituto de Investigacion Sanitaria San Carlos, Universidad Complutense de Madrid, Madrid, Spain
- Laboratory for Clinical Neuroscience, Center for Biomedical Technology, Universidad Politécnica de Madrid, IdISSC, Madrid, Spain
- Centre for Complex Interventions, Centre for Addiction and Mental Health, Toronto, ON, Canada
- Institute of Medical Sciences, University of Toronto, Toronto, ON, Canada
- Department of Psychiatry, University of Toronto, Toronto, ON, Canada
- Centre for Mental Health, Swinburne University, Melbourne, Australia
- Department of Psychiatry, University of Melbourne, Melbourne, Australia
- Department of Neurosurgery, Brigham & Women’s Hospital, Harvard Medical School, Boston, MA, USA
- NeuroCure Cluster of Excellence, Charité – Universitätsmedizin Berlin, Berlin, Germany
- Department of Psychiatry and Psychotherapy, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany
- Johanniter Hospital Oberhausen, EVKLN, Department of Psychiatry, Psychotherapy and Psychosomatics, Oberhausen, Germany
- Department of Stereotactic and Functional Neurosurgery, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany
- Univ Grenoble Alpes, Grenoble, France
- Inserm, U1216, Grenoble Institut des Neurosciences, Grenoble, France
- Department of Psychiatry, Icahn School of Medicine, Mount Sinai Hospital, New York, USA
- Krembil Brain Institute, Toronto, ON, Canada
- Psychiatry Department, CHU Grenoble Alpes, Grenoble, France
- Department of Neurology, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany
References
[1] Figee, M., & Mayberg, H. (2021). The future of personalized brain stimulation. Nature Medicine, 27, 196–197. https://doi.org/10.1038/s41591-021-01243-7
[2] Baldermann, J. C. et al. (2019). Connectivity profile predictive of effective deep brain stimulation in obsessive-compulsive disorder. Biological Psychiatry, 85(9), 735–743. https://doi.org/10.1016/j.biopsych.2018.12.019
[3] Baldermann, J. C. et al. (2021). Connectomic deep brain stimulation for obsessive-compulsive disorder. Biological Psychiatry, 90(10), 678–688. https://doi.org/10.1016/j.biopsych.2021.07.010
[4] Li, N. et al. (2020). A unified connectomic target for deep brain stimulation in obsessive-compulsive disorder. Nature Communications, 11, 3364. https://doi.org/10.1038/s41467-020-16734-3
[5] Hollunder, B. et al. (2022). Toward personalized medicine in connectomic deep brain stimulation. Progress in Neurobiology, 210(210), 102211. https://doi.org/10.1016/j.pneurobio.2021.102211
[6] Neudorfer, C. et al. Lead-DBS v3.0: Mapping deep brain stimulation effects to local anatomy and global networks. Neuroimage 268, 119862 (2023). https://doi.org/10.1016/j.neuroimage.2023.119862
[7] Irmen, F. et al. (2020). Left prefrontal connectivity links subthalamic stimulation with depressive symptoms. Annals of Neurology, 87(6), 962–975. https://doi.org/10.1002/ana.25734
Zvarova et al. (DBS Society Congress 2023)
A Novel Database Lookup Method for Deep Brain Stimulation Network Mapping
References
[1] Horn, A., Reich, M., Vorwerk, J., Li, N., Wenzel, G., Fang, Q., Schmitz-Hübsch, T., Nickl, R., Kupsch, A., Volkmann, J., Kühn, A.A., Fox, M.D., 2017. Connectivity Predicts deep brain stimulation outcome in Parkinson disease: DBS Outcome in PD. Ann Neurol. 82, 67–78. https://doi.org/10.1002/ana.24974
[2] Van Essen, D.C., Ugurbil, K., Auerbach, E., Barch, D., Behrens, T.E.J., Bucholz, R., Chang, A., Chen, L., Corbetta, M., Curtiss, S.W., Della Penna, S., Feinberg, D., Glasser, M.F., Harel, N., Heath, A.C., Larson-Prior, L., Marcus, D., Michalareas, G., Moeller, S., Oostenveld, R., Petersen, S.E., Prior, F., Schlaggar, B.L., Smith, S.M., Snyder, A.Z., Xu, J., Yacoub, E., 2012. The Human Connectome Project: A data acquisition perspective. Neuroimage 62, 2222–2231. https://doi.org/10.1016/j.neuroimage.2012.02.018
[3] Ewert, S., Plettig, P., Li, N., Chakravarty, M. M., Collins, D. L., Herrington, T. M., Kühn, A. A., & Horn, A. (2018). Toward defining deep brain stimulation targets in MNI space: A subcortical atlas based on multimodal MRI, histology and structural connectivity. NeuroImage, 170, 271–282. https://doi.org/10.1016/j.neuroimage.2017.05.015
Hollunder & Li et al. (Opto-DBS 2022)
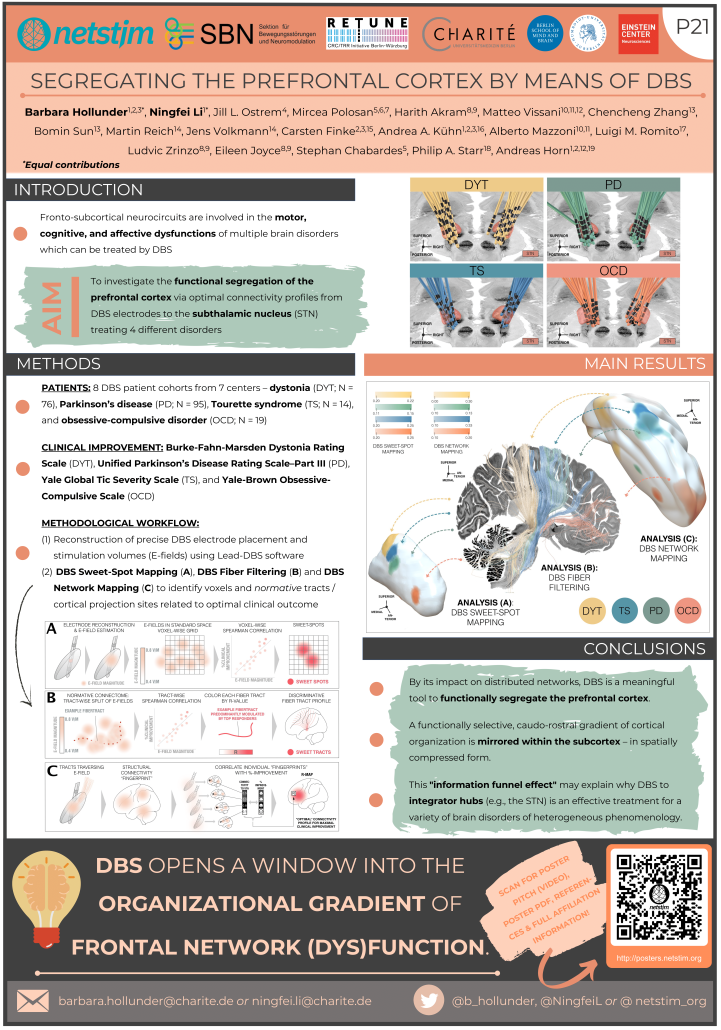
Segregating the Prefrontal Cortex by Means of DBS
Affiliations
- Movement Disorders and Neuromodulation Unit, Department of Neurology, Charité – Universitätsmedizin Berlin, Berlin, Germany
- Einstein Center for Neurosciences Berlin, Charité – Universitätsmedizin Berlin, Berlin, Germany
- Berlin School of Mind and Brain, Humboldt-Universität zu Berlin, Berlin, Germany
- Movement Disorders and Neuromodulation Centre, Department of Neurology, University of California San Francisco, San Francisco, CA, USA
- Université Grenoble Alpes, Grenoble, France
- Inserm, U1216, Grenoble Institut des Neurosciences, Grenoble, France
- Psychiatry Department, CHU Grenoble Alpes, Grenoble, France
- Department of Clinical and Movement Neurosciences, UCL Queen Square Institute of Neurology, London, UK
- National Hospital for Neurology and Neurosurgery, UCL Queen Square Institute of Neurology, London, UK
- The BioRobotics Institute, Scuola Superiore Sant’Anna, Pisa, Italy
- Department of Excellence in Robotics and AI, Scuola Superiore Sant’Anna, Pisa, Italy
- Department of Neurosurgery, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA
- Department of Neurosurgery, Rujin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- Department of Neurology, University Hospital Würzburg, Würzburg, Germany
- Department of Neurology, Charité – Universitätsmedizin Berlin, Berlin, Germany
- NeuroCure Cluster of Excellence, Charité – Universitätsmedizin Berlin, Berlin, Germany
- Parkinson and Movement Disorders Unit, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milan, Italy
- Department of Neurological Surgery, University of California San Francisco, San Francisco, CA, USA
- Center for Brain Circuit Therapeutics, Brigham & Women’s Hospital, Boston, MA, USA
References
Benarroch, E. E. (2008). Subthalamic nucleus and its connections: Anatomic substrate for the network effects of deep brain stimulation. Neurology, 70(21), 1991–1996. https://doi.org/10.1212/01.wnl.0000313022.39329.65
Bonelli, R. M., & Cummings, J. L. (2007). Frontal-subcortical circuitry and behavior. Dialogues in Clinical Neuroscience, 9(2), 141–151. https://doi.org/10.31887/dcns.2007.9.2/rbonelli
Ganos, C., Al-Fatly, B., Fischer, J.-F., Baldermann, J. C., Hennen, C., Visser-Vandewalle, V., … & Horn, A. (2022). A neural network for tics: insights from causal brain lesions and deep brain stimulation, Brain, awac009. https://doi.org/10.1093/brain/awac009
Haber, S. N., Liu, H., Seidlitz, J., & Bullmore, E. (2021). Prefrontal connectomics: From anatomy to human imaging. Neuropsychopharmacology. https://doi.org/10.1038/s41386-021-01156-6
Haynes, W. I. A., & Haber, S. N. (2013). The organization of prefrontal-subthalamic inputs in primates provides an anatomical substrate for both functional specificity and integration: Implications for basal ganglia models and deep brain stimulation. Journal of Neuroscience, 33(11), 4804–4814. https://doi.org/10.1523/JNEUROSCI.4674-12.2013
Horn, A., Li, N., Dembek, T. A., Kappel, A., Boulay, C., Ewert, S., Tietze, A., Husch, A., Perera, T., Neumann, W. J., Reisert, M., Si, H., Oostenveld, R., Rorden, C., Yeh, F. C., Fang, Q., Herrington, T. M., Vorwerk, J., & Kühn, A. A. (2019). Lead-DBS v2: Towards a comprehensive pipeline for deep brain stimulation imaging. NeuroImage, 184(July 2018), 293–316. https://doi.org/10.1016/j.neuroimage.2018.08.068
Horn, A., Reich, M., Vorwerk, J., Li, N., Wenzel, G., Fang, Q., Schmitz-Hübsch, T., Nickl, R., Kupsch, A., Volkmann, J., Kühn, A. A., & Fox, M. D. (2017). Connectivity predicts deep brain stimulation outcome in Parkinson disease. Annals of Neurology, 82(1), 67–78. https://doi.org/10.1002/ana.24974
Li, N., Baldermann, J. C., Kibleur, A., Treu, S., Akram, H., Elias, G. J. B., Boutet, A., Lozano, A. M., Al-Fatly, B., Strange, B., Barcia, J. A., Zrinzo, L., Joyce, E., Chabardes, S., Visser-Vandewalle, V., Polosan, M., Kuhn, J., Kühn, A. A., & Horn, A. (2020). A unified connectomic target for deep brain stimulation in obsessive-compulsive disorder. Nature Communications, 11, 3364. https://doi.org/10.1038/s41467-020-16734-3
Li, N., Hollunder, B., Baldermann, J. C., Kibleur, A., Treu, S., Akram, H., Al-Fatly, B., Strange, B. A., Barcia, J. A., Zrinzo, L., Joyce, E. M., Chabardes, S., Visser-Vandewalle, V., Polosan, M., Kuhn, J., Kühn, A. A., & Horn, A. (2021). A unified functional network target for deep brain stimulation in obsessive-compulsive disorder. Biological Psychiatry, 90(10), 701–713. https://doi.org/10.1016/j.biopsych.2021.04.006
Wang, F., Dong, Z., Tian, Q., Liao, C., Fan, Q., Hoge, W. S., Keil, B., Polimeni, J. R., Wald, L. L., Huang, S. Y., & Setsompop, K. (2021). In vivo human whole-brain Connectom diffusion MRI dataset at 760 µm isotropic resolution. Scientific Data, 8, 122. https://doi.org/10.1038/s41597-021-00904-z
Hollunder & Li et al. (OHBM 2022)
Segregating the prefrontal cortex by means of DBS
Affiliations
- Movement Disorders and Neuromodulation Unit, Department of Neurology, Charité – Universitätsmedizin Berlin, Berlin, Germany
- Einstein Center for Neurosciences Berlin, Charité – Universitätsmedizin Berlin, Berlin, Germany
- Berlin School of Mind and Brain, Humboldt-Universität zu Berlin, Berlin, Germany
- Movement Disorders and Neuromodulation Centre, Department of Neurology, University of California San Francisco, San Francisco, CA, USA
- Université Grenoble Alpes, Grenoble, France
- Inserm, U1216, Grenoble Institut des Neurosciences, Grenoble, France
- Psychiatry Department, CHU Grenoble Alpes, Grenoble, France
- Department of Clinical and Movement Neurosciences, UCL Queen Square Institute of Neurology, London, UK
- National Hospital for Neurology and Neurosurgery, UCL Queen Square Institute of Neurology, London, UK
- The BioRobotics Institute, Scuola Superiore Sant’Anna, Pisa, Italy
- Department of Excellence in Robotics and AI, Scuola Superiore Sant’Anna, Pisa, Italy
- Department of Neurosurgery, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA
- Department of Neurosurgery, Rujin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- Department of Neurology, University Hospital Würzburg, Würzburg, Germany
- Department of Neurology, Charité – Universitätsmedizin Berlin, Berlin, Germany
- NeuroCure Cluster of Excellence, Charité – Universitätsmedizin Berlin, Berlin, Germany
- Parkinson and Movement Disorders Unit, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milan, Italy
- Department of Neurological Surgery, University of California San Francisco, San Francisco, CA, USA
- Center for Brain Circuit Therapeutics, Brigham & Women’s Hospital, Boston, MA, USA
References
Benarroch, E. E. (2008). Subthalamic nucleus and its connections: Anatomic substrate for the network effects of deep brain stimulation. Neurology, 70(21), 1991–1996. https://doi.org/10.1212/01.wnl.0000313022.39329.65
Bonelli, R. M., & Cummings, J. L. (2007). Frontal-subcortical circuitry and behavior. Dialogues in Clinical Neuroscience, 9(2), 141–151. https://doi.org/10.31887/dcns.2007.9.2/rbonelli
Ganos, C., Al-Fatly, B., Fischer, J.-F., Baldermann, J. C., Hennen, C., Visser-Vandewalle, V., … & Horn, A. (2022). A neural network for tics: insights from causal brain lesions and deep brain stimulation, Brain, awac009. https://doi.org/10.1093/brain/awac009
Haber, S. N., Liu, H., Seidlitz, J., & Bullmore, E. (2021). Prefrontal connectomics: From anatomy to human imaging. Neuropsychopharmacology. https://doi.org/10.1038/s41386-021-01156-6
Haynes, W. I. A., & Haber, S. N. (2013). The organization of prefrontal-subthalamic inputs in primates provides an anatomical substrate for both functional specificity and integration: Implications for basal ganglia models and deep brain stimulation. Journal of Neuroscience, 33(11), 4804–4814. https://doi.org/10.1523/JNEUROSCI.4674-12.2013
Horn, A., Li, N., Dembek, T. A., Kappel, A., Boulay, C., Ewert, S., Tietze, A., Husch, A., Perera, T., Neumann, W. J., Reisert, M., Si, H., Oostenveld, R., Rorden, C., Yeh, F. C., Fang, Q., Herrington, T. M., Vorwerk, J., & Kühn, A. A. (2019). Lead-DBS v2: Towards a comprehensive pipeline for deep brain stimulation imaging. NeuroImage, 184(July 2018), 293–316. https://doi.org/10.1016/j.neuroimage.2018.08.068
Horn, A., Reich, M., Vorwerk, J., Li, N., Wenzel, G., Fang, Q., Schmitz-Hübsch, T., Nickl, R., Kupsch, A., Volkmann, J., Kühn, A. A., & Fox, M. D. (2017). Connectivity predicts deep brain stimulation outcome in Parkinson disease. Annals of Neurology, 82(1), 67–78. https://doi.org/10.1002/ana.24974
Li, N., Baldermann, J. C., Kibleur, A., Treu, S., Akram, H., Elias, G. J. B., Boutet, A., Lozano, A. M., Al-Fatly, B., Strange, B., Barcia, J. A., Zrinzo, L., Joyce, E., Chabardes, S., Visser-Vandewalle, V., Polosan, M., Kuhn, J., Kühn, A. A., & Horn, A. (2020). A unified connectomic target for deep brain stimulation in obsessive-compulsive disorder. Nature Communications, 11, 3364. https://doi.org/10.1038/s41467-020-16734-3
Li, N., Hollunder, B., Baldermann, J. C., Kibleur, A., Treu, S., Akram, H., Al-Fatly, B., Strange, B. A., Barcia, J. A., Zrinzo, L., Joyce, E. M., Chabardes, S., Visser-Vandewalle, V., Polosan, M., Kuhn, J., Kühn, A. A., & Horn, A. (2021). A unified functional network target for deep brain stimulation in obsessive-compulsive disorder. Biological Psychiatry, 90(10), 701–713. https://doi.org/10.1016/j.biopsych.2021.04.006
Wang, F., Dong, Z., Tian, Q., Liao, C., Fan, Q., Hoge, W. S., Keil, B., Polimeni, J. R., Wald, L. L., Huang, S. Y., & Setsompop, K. (2021). In vivo human whole-brain Connectom diffusion MRI dataset at 760 µm isotropic resolution. Scientific Data, 8, 122. https://doi.org/10.1038/s41597-021-00904-z
Goede et al. (DBS Expert Summit 2022)
DBS for Tremor: Network Effects
Goede et al. (DGKN 2022)
DBS for Tremor: Network Effects
Affiliations
Lukas L. Goede1,5, Bassam Al-Fatly1, Clemens Neudorfer4, Ningfei Li1, Vincent J.J. Odekerken2, Martin Reich3, Jens Volkmann3, Rob M.A. de Bie2, Andrea A. Kühn1, Andreas Horn1,4
1 Movement Disorder and Neuromodulation Unit, Department of Neurology, Charité Campus Mitte, Charité – Universitätsmedizin Berlin, Berlin, Germany
2 Department of Neurology, Amsterdam University Medical Center, Amsterdam, The Netherlands
3 Department of Neurology, University Clinic of Würzburg, Würzburg, Germany
4 Center for Brain Circuit Therapeutics, Department of Neurology Brigham & Women’s Hospital, Harvard Medical School, Boston, United States; MAMGH Neurosurgery & Center for Neurotechnology and Neurorecovery (CNTR) at MGH Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States
5 BIH – Berlin Institute of Health at Charité – Universitätsmedizin Berlin,
BIH Biomedical Innovation Academy, BIH Charité Junior Clinician Scientist Program, Charitéplatz 1, 10117 Berlin, Germany
References
Deuschl G, Bain P, Brin M. Consensus Statement of the Movement Disorder Society on Tremor. Mov Disord. 2008 Oct 20;13(S3):2–23.
Horn A, Li N, Dembek TA, Kappel A, Boulay C, Ewert S, et al. Lead-DBS v2: Towards a comprehensive pipeline for deep brain stimulation imaging. NeuroImage. 2019 Jan;184:293–316.
Horn A, Reich M, Vorwerk J, Li N, Wenzel G, Fang Q, et al. Connectivity Predicts deep brain stimulation outcome in Parkinson disease: DBS Outcome in PD. Ann Neurol. 2017 Jul;82(1):67–78.
Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BTT. The organization of the human cerebellum estimated by intrinsic functional connectivity. Journal of Neurophysiology. 2011 Nov;106(5):2322–45.
Middlebrooks EH, Okromelidze L, Wong JK, et al. Connectivity Correlates Predicting Deep Brain Stimulation Outcome in Essential Tremor: Evidence for a common treatment pathway. NeuroImage Clin. 2021.
Hollunder & Li et al. (DBS Expert Summit 2022)
Segregating the prefrontal cortex by means of DBS
References
Benarroch, E. E. (2008). Subthalamic nucleus and its connections: Anatomic substrate for the network effects of deep brain stimulation. Neurology, 70(21), 1991–1996. https://doi.org/10.1212/01.wnl.0000313022.39329.65
Bonelli, R. M., & Cummings, J. L. (2007). Frontal-subcortical circuitry and behavior. Dialogues in Clinical Neuroscience, 9(2), 141–151. https://doi.org/10.31887/dcns.2007.9.2/rbonelli
Ganos, C., Al-Fatly, B., Fischer, J.-F., Baldermann, J. C., Hennen, C., Visser-Vandewalle, V., … & Horn, A. (2022). A neural network for tics: insights from causal brain lesions and deep brain stimulation, Brain, awac009. https://doi.org/10.1093/brain/awac009
Haber, S. N., Liu, H., Seidlitz, J., & Bullmore, E. (2021). Prefrontal connectomics: From anatomy to human imaging. Neuropsychopharmacology. https://doi.org/10.1038/s41386-021-01156-6
Haynes, W. I. A., & Haber, S. N. (2013). The organization of prefrontal-subthalamic inputs in primates provides an anatomical substrate for both functional specificity and integration: Implications for basal ganglia models and deep brain stimulation. Journal of Neuroscience, 33(11), 4804–4814. https://doi.org/10.1523/JNEUROSCI.4674-12.2013
Horn, A., Li, N., Dembek, T. A., Kappel, A., Boulay, C., Ewert, S., Tietze, A., Husch, A., Perera, T., Neumann, W. J., Reisert, M., Si, H., Oostenveld, R., Rorden, C., Yeh, F. C., Fang, Q., Herrington, T. M., Vorwerk, J., & Kühn, A. A. (2019). Lead-DBS v2: Towards a comprehensive pipeline for deep brain stimulation imaging. NeuroImage, 184(July 2018), 293–316. https://doi.org/10.1016/j.neuroimage.2018.08.068
Horn, A., Reich, M., Vorwerk, J., Li, N., Wenzel, G., Fang, Q., Schmitz-Hübsch, T., Nickl, R., Kupsch, A., Volkmann, J., Kühn, A. A., & Fox, M. D. (2017). Connectivity predicts deep brain stimulation outcome in Parkinson disease. Annals of Neurology, 82(1), 67–78. https://doi.org/10.1002/ana.24974
Li, N., Baldermann, J. C., Kibleur, A., Treu, S., Akram, H., Elias, G. J. B., Boutet, A., Lozano, A. M., Al-Fatly, B., Strange, B., Barcia, J. A., Zrinzo, L., Joyce, E., Chabardes, S., Visser-Vandewalle, V., Polosan, M., Kuhn, J., Kühn, A. A., & Horn, A. (2020). A unified connectomic target for deep brain stimulation in obsessive-compulsive disorder. Nature Communications, 11, 3364. https://doi.org/10.1038/s41467-020-16734-3
Li, N., Hollunder, B., Baldermann, J. C., Kibleur, A., Treu, S., Akram, H., Al-Fatly, B., Strange, B. A., Barcia, J. A., Zrinzo, L., Joyce, E. M., Chabardes, S., Visser-Vandewalle, V., Polosan, M., Kuhn, J., Kühn, A. A., & Horn, A. (2021). A unified functional network target for deep brain stimulation in obsessive-compulsive disorder. Biological Psychiatry, 90(10), 701–713. https://doi.org/10.1016/j.biopsych.2021.04.006
Wang, F., Dong, Z., Tian, Q., Liao, C., Fan, Q., Hoge, W. S., Keil, B., Polimeni, J. R., Wald, L. L., Huang, S. Y., & Setsompop, K. (2021). In vivo human whole-brain Connectom diffusion MRI dataset at 760 µm isotropic resolution. Scientific Data, 8, 122. https://doi.org/10.1038/s41597-021-00904-z
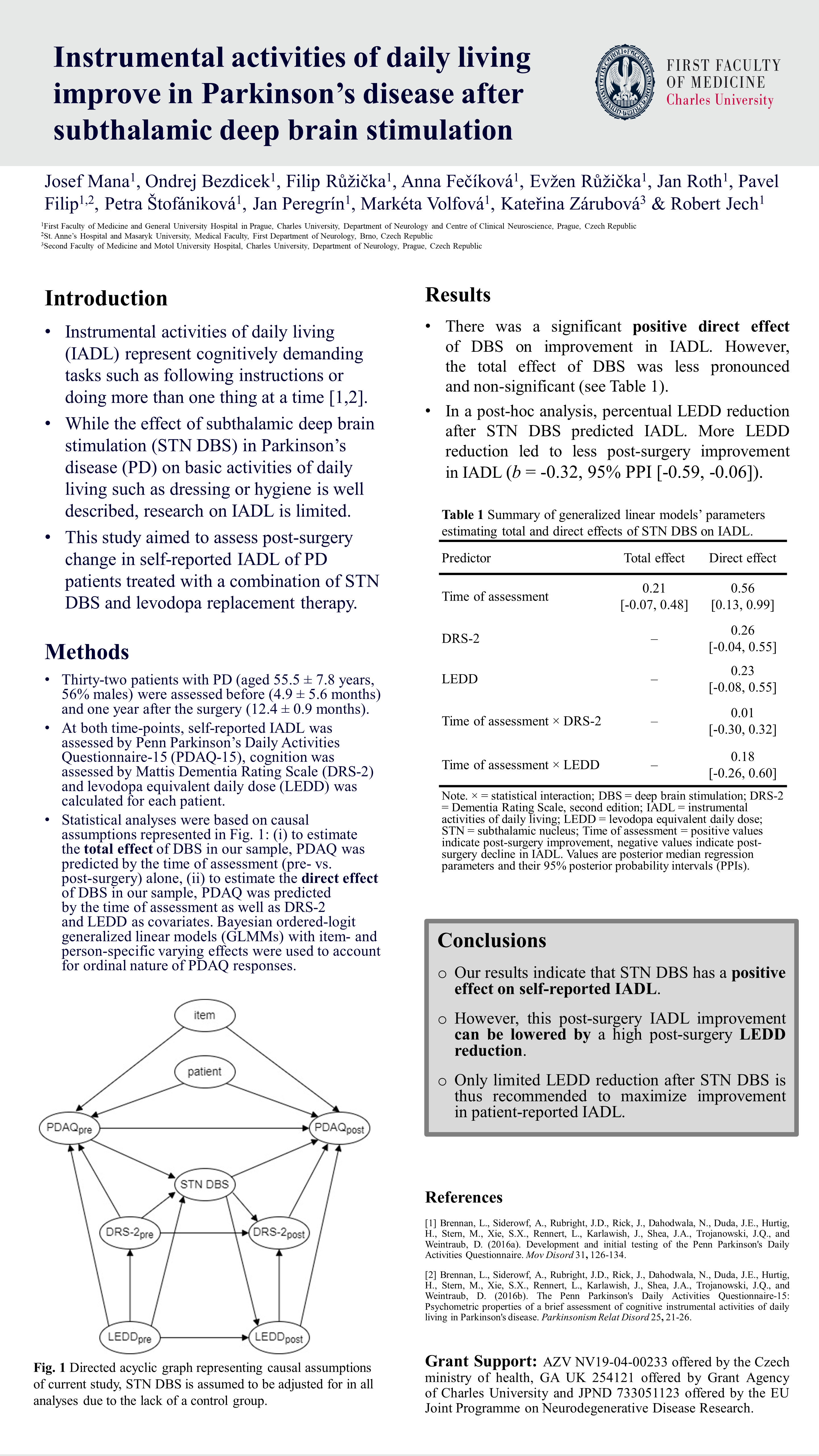
Mana et al. (DBS Expert Summit 2022)
Instrumental activities of daily living improve in Parkinson’s Disease after STN DBS
References
Brennan, L., Siderowf, A., Rubright, J.D., Rick, J., Dahodwala, N., Duda, J.E., Hurtig, H., Stern, M., Xie, S.X., Rennert, L., Karlawish, J., Shea, J.A., Trojanowski, J.Q., and Weintraub, D. (2016a). Development and initial testing of the Penn Parkinson’s Daily Activities Questionnaire. Mov Disord 31, 126-134. https://doi.org/10.1002/mds.2633
Brennan, L., Siderowf, A., Rubright, J.D., Rick, J., Dahodwala, N., Duda, J.E., Hurtig, H., Stern, M., Xie, S.X., Rennert, L., Karlawish, J., Shea, J.A., Trojanowski, J.Q., and Weintraub, D. (2016b). The Penn Parkinson’s Daily Activities Questionnaire-15: Psychometric properties of a brief assessment of cognitive instrumental activities of daily living in Parkinson’s disease. Parkinsonism Relat Disord 25, 21-26. https://doi.org/10.1016/j.parkreldis.2016.02.020
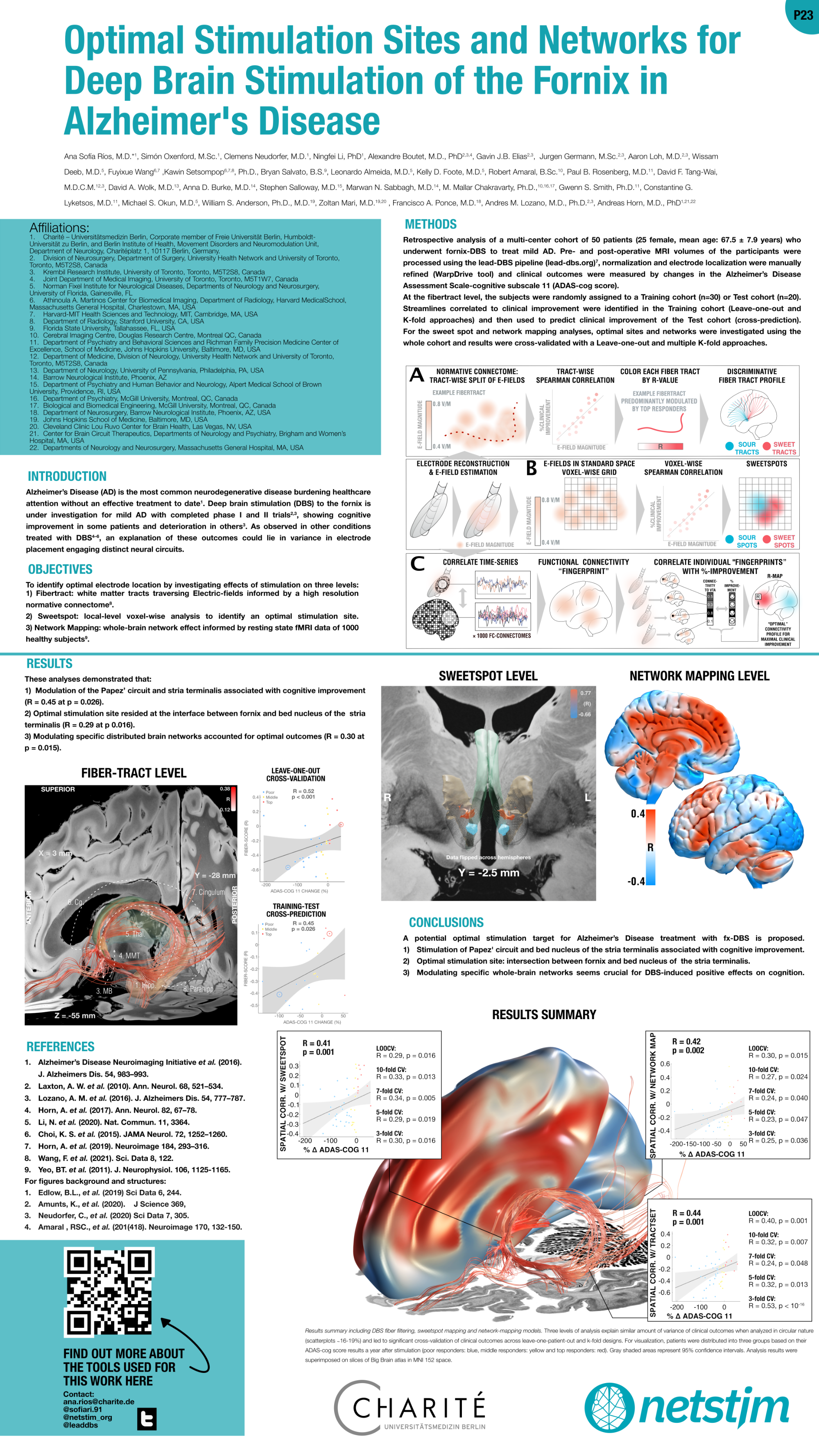
Rios et al. (DBS Expert Summit 2022)
Optimal stimulation sites and networks for DBS of the fornix in Alzheimer’s Diesease
References
Chen, Guangyu et al. “Staging Alzheimer’s Disease Risk by Sequencing Brain Function and Structure, Cerebrospinal Fluid, and Cognition Biomarkers.” Journal of Alzheimer’s disease : JAD vol. 54,3 (2016): 983-993. doi:10.3233/JAD-160537
Laxton, Adrian W et al. “A phase I trial of deep brain stimulation of memory circuits in Alzheimer’s disease.” Annals of neurology vol. 68,4 (2010): 521-34. doi:10.1002/ana.22089
Lozano, Andres M et al. “A Phase II Study of Fornix Deep Brain Stimulation in Mild Alzheimer’s Disease.” Journal of Alzheimer’s disease : JAD vol. 54,2 (2016): 777-87. doi:10.3233/JAD-160017
Horn, Andreas et al. “Connectivity Predicts deep brain stimulation outcome in Parkinson disease.” Annals of neurology vol. 82,1 (2017): 67-78. doi:10.1002/ana.24974
Li, Ningfei et al. “A unified connectomic target for deep brain stimulation in obsessive-compulsive disorder.” Nature communicationsvol. 11,1 3364. 3 Jul. 2020, doi:10.1038/s41467-020-16734-3
Choi, Ki Sueng et al. “Mapping the “Depression Switch” During Intraoperative Testing of Subcallosal Cingulate Deep Brain Stimulation.” JAMA neurology vol. 72,11 (2015): 1252-60. doi:10.1001/jamaneurol.2015.2564
Horn, Andreas et al. “Lead-DBS v2: Towards a comprehensive pipeline for deep brain stimulation imaging.” NeuroImage vol. 184 (2019): 293-316. doi:10.1016/j.neuroimage.2018.08.068
Wang, Fuyixue et al. “In vivo human whole-brain Connectom diffusion MRI dataset at 760 µm isotropic resolution.” Scientific data vol. 8,1 122. 29 Apr. 2021, doi:10.1038/s41597-021-00904-z
Yeo, B T Thomas et al. “The organization of the human cerebral cortex estimated by intrinsic functional connectivity.” Journal of neurophysiology vol. 106,3 (2011): 1125-65. doi:10.1152/jn.00338.2011
For figure background and structures
Edlow, Brian L et al. “7 Tesla MRI of the ex vivo human brain at 100 micron resolution.” Scientific data vol. 6,1 244. 30 Oct. 2019, doi:10.1038/s41597-019-0254-8
Amunts, Katrin et al. “Julich-Brain: A 3D probabilistic atlas of the human brain’s cytoarchitecture.” Science (New York, N.Y.)vol. 369,6506 (2020): 988-992. doi:10.1126/science.abb4588
Neudorfer, Clemens et al. “A high-resolution in vivo magnetic resonance imaging atlas of the human hypothalamic region.” Scientific datavol. 7,1 305. 15 Sep. 2020, doi:10.1038/s41597-020-00644-6
Amaral, Robert S C et al. “Manual segmentation of the fornix, fimbria, and alveus on high-resolution 3T MRI: Application via fully-automated mapping of the human memory circuit white and grey matter in healthy and pathological aging.” NeuroImage vol. 170 (2018): 132-150. doi:10.1016/j.neuroimage.2016.10.027